PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
1.7
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions
APPLICATIONS
APPEARANCE
ASSAY
98.0% min
MELTING POINT
28 - 30 C
|
|
|
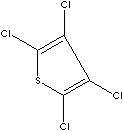
| TETRACHLOROTHIOPHENE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 6012-97-1 |
|
| EINECS NO. | 227-866-4 | |
| FORMULA | C4Cl4S | |
| MOL WT. | 221.92 | |
|
H.S. CODE |
||
|
TOXICITY |
||
| SYNONYMS | Penphene; Pemphene; 2,3,4,5-Tetrachlorothiophene; | |
| Perchlorothiophene; Tetrachlorothiofene; TCTP; 2,3,4,5-Chlorothiophene; Tetrachlorthiofen; Tetraclorotiofeno; Tétrachlorothiophène; | ||
| DERIVATION |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to yellow liquid | |
| MELTING POINT | 28 - 30 C | |
| BOILING POINT | 75 C at 2 mmHg | |
| SPECIFIC GRAVITY |
1.7 | |
| SOLUBILITY IN WATER | Insoluble | |
|
SOLVENT SOLUBILITY |
||
| pH | ||
| VAPOR PRESSURE | ||
|
REFRACTIVE INDEX |
1.591 | |
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 2; Flammability: 3; Reactivity: 0 | |
| FLASH POINT | 22 C | |
| STABILITY |
Stable under ordinary conditions | |
|
APPLICATIONS |
||
| Thiophene, also known as thiofuran, is a cyclic compound containing four carbon atoms and one sulfur atom in the ring. Thiophene is an analog to furan and pyrrole where the sulfur atom is replaced by O and NH respectively. Thiophene is a toxic, flammable, and colorless liquid; insoluble in water (soluble in most organic solvents including alcohol and ether); melting at -38 C, boiling at 84 C. Thiophene is the simplest aromatic compound containing sulfur atom and it shares some similar chemical properties with benzene. The lone electron pairs on sulfur in the delocalized pi electron system does not exhibits the properties of thioethers but aromaticity. The sulfur atom is unreactive but the adjacent carbons are susceptible to attack by electrophiles. It is reactive toward sulfonation. In commercial thiophene can be prepared by the reaction of butane and sulfur. Thiophenes are also prepared by the reaction of diketones with Lawesson's reagent. Thiophene and its derivatives exist in petroleum or coal. Thiophene derivatives are also found in natural plant pigments. Biotin, a water-soluble B-complex vitamin, is a reduced thiophene derivative. Thiophene moiety is found in ccphalothin antiboitics. Thiophene is used as a solvent and chemical intermediate. Its derivatives are used in manufacturing dyes, aroma compounds and pharmaceuticals. They are used as monomers to make condensation copolymers. Organic conductive polymers are responsible for the important materials science for the application of polymer electro luminescence. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellow to yellowish liquid | |
|
ASSAY |
98.0% min |
|
|
MELTING POINT |
28 - 30 C |
|
| TRANSPORTATION | ||
| PACKING |
| |
| HAZARD CLASS | 6.1 (Packing Group: III) | |
| UN NO. | 2811 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T, Risk Phrases: 23/24/25, Safety Phrases: 26-28-36/37/39-45 | ||
| PRICE INFORMATION | ||
|
|
|