| CAS
NO. |
14345-97-2 |
|
| EINECS NO. |
238-296-0 |
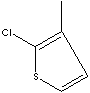
| FORMULA |
C5H5ClS |
| MOL
WT. |
132.61 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
2-cloro-3-metiltiofeno (Spanish);
|
| 2-Chlor-3-methylthiophen (German);
2-chloro-3-méthylthiophène (French); |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
clear to yellow
liquid |
| MELTING POINT |
|
| BOILING
POINT |
147
- 150 C |
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
Health: 1; Flammability: 0; Reactivity: 0 |
|
REFRACTIVE
INDEX
|
1.5395 - 1.5415 |
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
GENERAL
DESCRIPTION AND APPLICATIONS
|
|
Thiophene, also known as thiofuran, is a cyclic compound containing four carbon
atoms and one sulfur atom in the ring. Thiophene is an analog to furan and pyrrole where the sulfur
atom is replaced by O and NH
respectively. Thiophene is a toxic, flammable, and colorless liquid; insoluble in water (soluble in most
organic solvents including alcohol and
ether); melting at -38 C, boiling at 84 C. Thiophene is the simplest aromatic
compound containing sulfur atom and it shares some similar
chemical properties with benzene. The lone electron pairs on sulfur in the delocalized pi electron system does
not exhibits the properties of thioethers but aromaticity.
The sulfur atom is unreactive but the adjacent carbons
are susceptible to attack
by electrophiles. It is reactive toward sulfonation. In commercial thiophene
can be prepared by the reaction of butane and sulfur.
Thiophenes are also prepared by the reaction of diketones with Lawesson's
reagent. Thiophene and its derivatives exist in petroleum or coal. Thiophene derivatives
are also found in natural plant pigments. Biotin, a water-soluble B-complex vitamin,
is a reduced thiophene derivative. Thiophene moiety
is found in ccphalothin antiboitics. Thiophene is used as a solvent and chemical intermediate. Its
derivatives are used in manufacturing dyes, aroma compounds and
pharmaceuticals. They are used as monomers to make
condensation copolymers. Organic conductive polymers are responsible for the
important materials science for the application of polymer
electro
luminescence. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
clear to yellow
liquid |
|
ASSAY
|
98.0%
min
|
|
WATER
|
0.5%
max
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Hazard
Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26-37/39 |
|
PRICE
INFORMATION
|
|

|
