| CAS
NO. |
53994-73-3
(Base)
70356-03-5 (Monohydrate) |
|
| EINECS NO. |
258-909-5 |
| FORMULA |
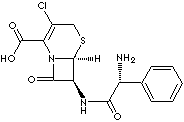
C15H14ClN3O4S·H2O |
| MOL
WT. |
385.82 |
|
H.S.
CODE
|
2941.90 |
|
TOXICITY
|
|
| SYNONYMS |
3-Chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid; |
| (6R-(6a,7beta(R')))-7-((aminophenylacetyl)amino)-3-chloro-
8-oxo-5-thia-1-azabicyclo(4.2.0) oct-2-ene-2-carboxylic acid; |
|
CLASSIFICATION
|
ANTIBOITICS
/ CEPHALOSPORINS
/
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
White to off-white
crystalline powder |
| MELTING POINT |
|
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
|
Cefaclor is a semisynthetic 2nd generation cephalosporin antibiotic inhibiting
cell wall biosynthesis. It is used in the treatment of respiratory and urinary
tract infections and and of the skin and soft tissues; administered orally.
Cephalosporins differ from penicillins in the structure of the bicyclic ring
system. Chemical designation is
(6R-(6a,7beta(R')))-7-((amino phenyl acetyl)amino)-3- chloro-
8-oxo-5-thia-1-azabicyclo (4.2.0)oct-2-ene-2-carboxylic acid. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
White to off-white
crystalline powder |
|
IDENTIFICATION
|
Complies
Test A,B
|
|
ASSAY |
950
- 1020 ug/mg |
|
MOISTURE |
3.0%
- 6.5% |
|
pH
|
3.0
- 4.5 |
|
IMPURITY
|
0.5%
max (individual), 2.0% max (sum)
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Price:

|
