| CAS
NO. |
92665-29-7 |
|
| EINECS
NO. |
|
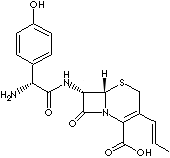
| FORMULA |
C18H19N3O5S·H2O |
| MOL
WT. |
347.39 |
|
H.S.
CODE
|
|
|
TOXICITY |
|
| SYNONYMS |
Cefzil; |
|
(6R,7R)-7-((R)-2-amino-2-(p-hydroxy-phenyl)acetamido)-8-oxo-3-propenyl-5-thia-1-
azabicyclo (4,2,0) oct-2-ene-2-carboxylic
acid monohydrate; |
|
CLASSIFICATION
|
ANTIBOITICS
/ CEPHALOSPORINS
/
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
to off-white powder |
| MELTING
POINT |
|
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR
DENSITY |
|
| AUTOIGNITION |
|
| NFPA
RATINGS |
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
| Cefalexin is a semisynthetic 2nd generation cephalosporin antibiotic inhibiting
cell wall biosynthesis. It is used especially in the treatment of respiratory
and urinary tract infections and is intended for oral administration.
Cephalosporins differ from penicillins in the structure of the bicyclic ring
system. Cefprozil
is chemically (6R,7R)-7-((R)-2-amino-2-(p-hydroxy-phenyl)
acetamido)-8-oxo-3-propenyl-5-thia-1- azabicyclo(4.2.0)oct-2-ene-2-carboxylic
acid monohydrate. Commercial cefalexin is available as a mixture of cis- and
trans form (cis: 90%) and in a monohydrate form which is a white
to yellowish crystalline powder with a bitter taste and low solubility in water. |
| SALES
SPECIFICATION (USP, BP) |
|
APPEARANCE
|
white
to yellow crystalline powder |
|
CONTENT
|
98.0
- 101.0% (On dry basis)
|
|
WATER
|
3.5
- 6.0%
|
|
pH |
4.8 |
|
ISOMER
RATIO
|
0.09
|
| TRANSPORTATION |
| PACKING |
25kgs
in fiber drum
|
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
SHELF-LIFE:
5 years
Price:
 |
