PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
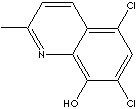
Chlorquinaldol, 5,7-dichloro-2-methyl-8-hydroxyquinoline, has antibacterial, antifungal and amoebicidal activity with antieczematic and antipruritic properties; used topically in the treatment of cutaneous and vaginal infections. It is a similar agent to clioquinol.
Clioquinol is one of the halogenated 8-quinolinols which are antibacterial, intestinal antiamebic, and vaginal trichomonacide agents. They are derived from 8-hydroxyquinoline, an antiseptic agent with mild fungistatic, bacteriostatic, anthelmintic, and amebicidal action. Clioquinol, iodochlorohydroxyquinoline, is an antifungal with antieczematic and antipruritic properties. Clioquinol topical preparations are used to treat skin infections. It was administered orally in the treatment of amebic dysentery. The oral preparation is banned worldwide due to associated subacute myelo-optic neuropathy.
APPEARANCE
CONTENT
99.0% min
MELTING POINT
25kgs
in fiber drum
PRICE INFORMATION