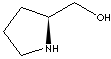
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
1.025
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
APPLICATIONS
Chiral amino alcohol possessing both amine and alcohol is a structural motif for the compounds which have important biological and pharmacological functions within the body as inhibitor of aspartyl proteases, aldose reductase, b-amyloid peptide formation, and dopamine D4 antagonists. The chiral amino alcohol also exhibits anti-inflammatory, anesthetic, antipasmodic, hepatotoxic, anti-diabetic, anti-obesity,and anti-depressant activities. Chiral amino alcohols and their N-protected derivatives (Cbz, t-Boc, and Fmoc) are versatile building blocks, auxiliaries, ligand or resolving agent in asymmetric synthesis for biological and pharmaceutical researches. applications are present in the field of antitumor, anesthetic, antipasmodic, hepatotoxic, antiinflammatory or anti-HIV activities. Their derivatives include the forms of;
- C-terminal peptibiols
- Amphiphylic antibacterial peptides
- Oligocarbamates
- Analogs of peptides
- Aziridines, Halogen aminoalkyls
- Enantiomerical alpha-alkyl mono and diamines
- Enantiomerical beta-substituted amines
- Enantiomerical beta-amino acids beta-amino sulfoxides, beta-amino sulfides and
- beta-amino thiols.
- The precursors of diverse compounds such as statines, sphingolipides and peptide isosteres.
APPEARANCE
ASSAY
99.0% min
Optical rotation
-29° ~ -32° (C=1 in toluene)