PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
TAXANES /
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
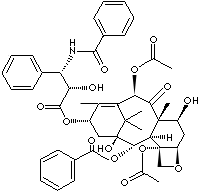
Paclitaxel, containing oxetane ring nucleus, is one of the most medicinally valuable diterpenes in which phyto and vitamin A1 are classified. It is an antineoplastic agent that acts by promoting and stabilizing the polymerization of microtubules. It is semi-synthetic or originally obtained from the Pacific yew tree (Taxus brevifolia). It is a white to off-white crystalline powder; insoluble in water; soluble in ethanol and acetone; melting point 216 - 217 C. The chemical designation is 5-beta,20-epoxy-1,2-alpha, 4,7-beta,10-beta,13-alpha- hexahydroxy-tax- 11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenyl Isoserine. Paclitaxel is commonly referred to Taxol, by the trade name. Paclitaxel is a representative compound belong to the family of compounds called taxanes. It binds to the N-terminal region of b-tubulin and promotes the formation of highly stable microtubules that resist depolymerization, and thus inhibits cell replication. It is used to treat cancers of ovaries, breast, head, neck and certain types of lung cancer, as well as cancers of skin and mucous membrane more commonly found in patients suffered from AIDS (acquired immunodeficiency syndrome). Other related taxanes modified by substitution of functional side chains including N-acyl groups, -OH group addition and stereochemistry for better solubility in water, oral bioavailability and cytotoxic activity include followings:
- 10-Deacetyl-7-epibaccatin III
- 10-Deacetyl-7-epipaclitaxel
- 10-Deacetyl-7-xylosylpaclitaxel C
- 10-Deacetyl-7-xylosylpaclitaxel ( CAS #: 90332-65-3)
- 10-Deacetylbaccatin III ( CAS #: 32981-86-5)
- 10-Deacetylcephalomannine
- 10-Deacetyl-N-Debenzoylpaclitaxel
- 10-Deacetylpaclitaxel
- 10-Deaceyl-7-xylosylpaclitaxel
- 13-Acetyl-9-dihydrobaccatin III ( CAS #: 142203-65-4)
- 2',3'-Dihydrocephalomannine (Paclitaxel E)
- 2-Debenzoylpaclitaxel
- 7-Epi-10-Deacetylcephalomannine
- 7-Epicephalomannine
- 7-Epipaclitaxel ( CAS #: 105454-04-4)
- 7-Xylosylpactlitaxel C
- 7-Xylosylpactlitaxel
- Baccatin III ( CAS #: 27548-93-2, (the form that side chains are removed)
- Cephalomannine (( CAS #: 71610-00-9, Paclitaxel B)
- Docetaxel ( CAS #: 114977-28-5)
- Isocephalomannine (2-Debenzoylpaclitaxel-2-Pentenoate)
- N-Benzoyl-(2R, 3S)-3-phenylisoserine ( CAS #: 132201-33-3, side chain in paclitaxel)
- N-Benzoyl-(2R, 3S)-3-phenylisoserine esters (side chain in paclitaxels)
- N-Debenzoylpaclitaxel
- N-Methylpaclitaxel C
- N-Methylpaclitaxel
- Paclitaxel C
- Paclitaxel D
- Paclitaxel F
- Paclitaxel G
APPEARANCE
white to off-white crystalline powder
IDENTIFICATION
pass
ASSAY (HPLC)
99.0 - 101.0%
OPTICAL ROTATION
-49° ~ -55°
SOLVENT RESIDUE
0.2% max
MELTING POINT
210 - 213 C
INDIVIDUAL IMPURITY
Cephalomanin: 0.1% max
Total Impurity: 0.2% max
LOSS ON DRYING
2.0% max
SULFATED ASH
0.1% max
3.0% max
HEAVY METALS
10ppm max
PRICE INFORMATION