ACETAZOLAMIDE
PRODUCT IDENTIFICATION
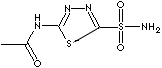
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
Acetazolamide is a potent carbonic anhydrase inhibitor,
effective in the control of fluid secretion (e.g., some types of glaucoma), in
the treatment of certain convulsive disorders (e.g., epilepsy) and in the
promotion of diuresis in instances of abnormal fluid retention (e.g., cardiac
edema).
Acetazolamide is not a mercurial diuretic. Rather, it is a
nonbacteriostatic sulfonamide possessing a chemical structure and
pharmacological activity distinctly different from the bacteriostatic
sulfonamides.
Acetazolamide is an enzyme inhibitor that acts specifically
on carbonic anhydrase, the enzyme that catalyzes the reversible reaction
involving the hydration of carbon dioxide and the dehydration of carbonic acid.
In the eye, this inhibitory action of Acetazolamide decreases the secretion of
aqueous humor and results in a drop in intraocular pressure, a reaction
considered desirable in cases of glaucoma and even in certain nonglaucomatous
conditions. Evidence seems to indicate that Acetazolamide has utility as an
adjuvant in the treatment of certain dysfunctions of the central nervous system
(e.g., epilepsy).
Inhibition of carbonic anhydrase in this area appears to
retard abnormal, paroxysmal, excessive discharge from central nervous system
neurons. The diuretic effect of Acetazolamide is due to its action in the kidney
on the reversible reaction involving hydration of carbon dioxide and dehydration
of carbonic acid.
The result is renal loss of HCO3 ion, which carries out sodium, water, and potassium.
Alkalinization of the urine and promotion of diuresis are thus effected.
Alteration in ammonia metabolism occurs due to increased reabsorption of ammonia
by the renal tubules as a result of urinary alkalinization.
http://www.drugs.com/
Acetazolamide is a carbonic anhydrase inhibitor. Carbonic anhydrase is a protein in your body. Acetazolamide reduces the activity of this protein. Acetazolamide is used to treat glaucoma and to treat and to prevent acute mountain sickness (altitude sickness). It is also used as a part of some treatment plans for congestive heart failure and seizure disorders. Acetazolamide may also be used for purposes other than those listed in this medication guide. (http://www.med.umich.edu/)
Acetazolamide belongs to a group of medicines called
carbonic anhydrase inhibitors.
Carbonic anhydrase is a chemical in the
body that is responsible for the production and breakdown of carbonic acid. Part
of this reaction results in the production of bicarbonate. Acetazolamide acts to
inhibit the action of carbonic anhydrase and thereby decrease the production of
bicarbonate
Bicarbonate is required for the production of the fluid that
fills the back of the eye (aqueous humour). By decreasing the production of
bicarbonate, acetazolamide decreases the amount of aqueous humour produced in
the eye. This helps reduce the pressure caused by the fluid within the eye in
conditions such as glaucoma.
In the kidneys, acetazolamide acts to
increase the amount of bicarbonate lost from the body. Bicarbonate draws water
with it from the kidneys. This results in a small increase in the amount of
water being lost from the body. Therefore acetazolamide has some activity as a
diuretic. As acetazolamide causes only minor water loss, it is called a weak
diuretic.
Acetazolamide may also be used in conjunction with other
anti-epileptic medicines in the treatment of epilepsy. It is not fully
understood how it works in this condition. It is thought to stabilise the
activity of nerves. Chemicals must pass through openings on nerve cells for
electrical signals to be generated. By decreasing the amount of bicarbonate
produced in the body, acetazolamide alters the balance of chemicals in the
blood. This may alter the amounts of some chemicals that can pass through the
openings on nerve cells. This in turn, may help prevent excessively rapid and
repetitive firing of electrical signals. Thereby electrical nerve activity in
the brain may be stabilised, leading to prevention of fits and maintenance of
normal brain function. http://www.netdoctor.co.uk/
APPEARANCE
ASSAY
99.0% min
258 - 261 C
SULFATE
0.3% max
LOSS ON DRYING
0.5% max
CHLORIDE
0.1% max
SULFATE ASH
0.2% max
RESIDUE ON IGNITION
0.1% max
RELATED SUBSTANCE
1.0% max
CLARITY OF SOLUTION