| 1,8-Dihydroxyanthrachinon;
1,8-dihydroxy-9,10-anthraquinone; Dioxyanthrachinonum;
Dihydroxyanthraquinone;
1,8-Dihydroxy-9,10-Anthracenedione; 1,4,5,8-Tetroxyantraquinone;
|
|
Anthraquinone, a polycyclic aromatic hydrocarbon containing two opposite
carbonyl groups (C=O) at 9,10 position, is yellow or light gray to gray-green
crystal powder; insoluble in water. , In nature, it occurs in plants (aloe,
cascara sagrada, senna, and rhubarb), fungi, lichens, and insects as a parent
material for coloring of yellow, orange, red, red-brown, or violet. It is
commercially produced by several ways including by oxidation of anthracene with
chromic acid, the condensation of benzene and phthalic anhydride, followed by
dehydration for cyclization, and diene Diels-Alder
reaction. Anthraquinone is the most important quinone derivative of anthracene
as the parent substance of a large class of dyes and pigments. One of the oldest
mordant dye, alizarin, is the anthraquinone derivative. Anthraquinone is a
starting material for the production of coloring compounds, antioxidants, and
polymerization inhibitors. Its derivatives are widely used as intermediates for
dyes, pigments, photographic chemicals, and paints. Anthraquinone is used in
paper industry as a catalyst to increase the pulp production yield and to
improves the fiber strength through reduction reaction of cellulose to
carboxylic acid. Natural anthraquinones have cathartic properties.
Anthraquinones derivatives are also used in manufacturing drugs. Mitoxantrone,
an
antineoplastic is an example. Quinizarin, 1,4-Dihydroxyanthraquinone, is used as an intermediate for the synthesis of
indanthrene and alizarin dyestuffs. It is also used to form lakes with calcium,
barium, and lead. Alizarin is 1,2-Dihydroxyanthraquinone.
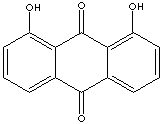
Chrysazin is
1,8-Dihydroxyanthraquinone.
Chrysazin is
called danthron when
used as a cathartic. It was used as a stimulant laxative to treat constipation. But it is no
longer widely used due to its potential to cause cancer. 2,6-Dihydroxyanthraquinone
is called anthraflavic Acid.
Dihydroxyanthraquinone
molecules include:
|