PRODUCT IDENTIFICATION
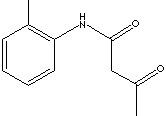
2924.29
Oral rat LD50: 1600 mg/kg
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
103 C
1.062
REFRACTIVE INDEX
APPLICATIONS
Acetoacetates have a reactive hydrogen atom on the carbon alpha to both carbonyl groups. It undergoes Knoevenagel condensation reaction as a reactant to forms a large class of target products including amino acids, drugs, colorants, lacquers, perfumes, and plastics. Alone, it is used as a flavoring agent and a solvent. Knoevenagel condensation is a nucleophilic addition of a reactive hydrogen atom at 1,3-diketone compounds to a carbonyl group, followed by an dehydration reaction. 1,3-Diketone compounds (beta-ketones) include malonic acid, diethyl malonate, Meldrum's acid, and acetoacetic acid derivatives.
AAOT is used in manufacturing agricultural chemicals, coating materials, dyes and pigments, pharmaceuticals, co-promoters for polymers. Anilide is a amide in which one or more hydrogens are replaced by phenyl; having the C6H5NH2-group.
APPEARANCE
White to off white powder
99.0% min
MELTING POINT
103 C min
MOISTURE
0.2% max
IRON
0.5ppm max