|
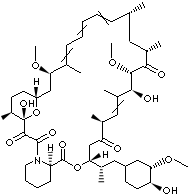
SIROLIMUS |
|
23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine; (-)-Rapamycin; Antibiotic AY 22989; Rapammune; Rapamune; Rapamycin; (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R, 27R,34aS)- 9,10,12,13,14,21,22,23,24,25,26,27, 32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-((1R)-2-((1S,3R,4R)- 4-hydroxy-3-methoxycyclohexyl)- 1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27- epoxy- 3H-pyrido(2,1-c)(1,4) oxaazacyclohentriacontine-1,5,11,28,29 (4H,6H,31H)pentone; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
53123-88-9 |
|
EINECS RN |
|
|
FORMULA |
C51H79NO13 |
|
MOLE WEIGHT |
914.17 |
|
SOURCE |
Streptomyces hygroscopicus |
|
CLASSIFICATION |
Antibiotic / Immunosuppressant / Macrolide compound |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
pale yellow crystalline power |
|
MELTING POINT |
183 - 185 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble (soluble in DMSO) |
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents. |
| DECOMPOSITION PRODUCTS |
Carbon oxides. nitrogen oxides. |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 1 Flammability: 1 Reactivity: 0 |
|
|
|
POTENTIAL HEALTH EFFECTS |
|
|
HAZARD NOTES |
Target Organs: Immune system |
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
May be harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
| TARGET ORGANS |
Immune system |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
22-24/25 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | |
|
Sirolimus (rapamycin, Rapamune®′) is an
immunosuppressant that inhibits cytokine-stimulated T-cell proliferation.
Sirolimus acts by forming a complex with FK-binding protein-12 which in turn
binds to mTOR kinase, a specific cell cycle regulatory protein, thereby
inhibiting mTOR action. mTOR inhibition prevents cell cycle progression from G1
to S phase in T-cells and, thus, T-cell proliferation. mTOR inhibition is a
different mechanism of action than that of calcineurin-inhibiting agents such as
cyclosporine (CsA), a fact that may account for the synergistic effect of
sirolimus/CsA combined therapy following renal transplant. Sirolimus is
currently recommended for use in conjunction with cyclosporine (and
corticosteroids) to reduce or prevent graft rejection by the host. Sirolimus
dose-related side effects include increased serum levels of cholesterol,
triglycerides, and creatinine and decreased glomerular filtration rate.
Hypertension, rash, anemia, arthralgia, diarrhea, hypokalemia, leukopenia, and
thrombocytopenia also may occur.
(http://www.questdiagnostics.com/)
Rapamycin, also known as sirolimus, is an FDA-approved antibiotic and immunosuppressant. It is already being used in organ transplant patients and is currently being tested in phase II and III clinical trials in cancer patients for its antitumor activity. Rapamycin inhibits the activity of a protein called mTOR which, among its other functions, inhibits a process called autophagy. Autophagy is the process by which a cell breaks down its own molecules and other components that are no longer needed. Since mTOR functions to inhibit autophagy, by inhibiting mTOR, rapamycin promotes autophagy, allowing for the breakdown of unnecessary components of the cell. Researchers have shown in fly and mouse models of HD that by inducing autophagy, rapamycin helps nerve cells break down huntingtin aggregates. Whether these protein aggregates are a cause or result of the HD disease process is not yet known. However, nerve cells that build up huntingtin aggregates in the brains of people with HD often die. (To read more about huntingtin protein aggregation and its role in HD, click here.) Thus, rapamycin may help prevent cell death by helping nerve cells clear out huntingtin aggregates. Rapamycin could be an especially promising treatment if started before or shortly after the onset of symptoms in people with HD, when the levels of huntingtin aggregates in the nerve cells are still manageable. (http://www.stanford.edu/) Pharmacological actions:
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to yellow crystalline powder |
|
IDENTIFICATION |
pass Test A,B,C |
|
ASSAY |
98.0 - 102.0% |
|
STEROISOMER |
cis-Sirolimus 5.0% max |
|
SOLVENT RESIDUES |
Ethanol :5000ppm max |
|
IMPURITIES |
Individual impurity: 1.0% max |
|
LOSS ON DRYING |
0.5% max |
|
HEAVY METALS |
20ppm max |
|
|
| PRICE INFORMATION |
|
|