PRODUCT IDENTIFICATION
52-51-7
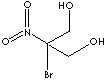
199.98
HS CODE
TOXICITY
CLASSIFICATION
Non-glutaraldehyde-based Biocide
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
130 - 133C
SOLUBILITY IN WATER
22g/100ml
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
130 C
APPLICATIONS
Formaldehyde is the simplest aldehyde with the formula HCH=O. It is a gas at room temperature with a pungent smell. It cannot be readily isolated or handled in the pure state because it is extremely reactive. Formaldehyde reversibly polymerizes to its cyclic trimer 1,3,5-trioxane or the linear polymer polyoxymethylene. It is readily oxidized to form formic acid in the air. It is commercially available as an aqueous solution usually 37-50% w/w. Formalin is the solution of formaldehyde in water. Paraformaldehyde is a white crystalline solid formed by polymerization of formaldehyde. Formaldehyde kills most bacteria, and used as a disinfectant and as a preservative and fixative for pathologic specimens. Formaldehyde cross links amino groups. In industry formaldehyde is used in the production of thermoset resins, functional polyols, explosive, hexamine and other chemicals. Examples of formaldehyde-releasing biocides are:
- Imidazolidinyl Urea (CAS RN: 39236-46-9)
- Diazolidinyl Urea (CAS RN: 78491-02-8)
- 1,3-Dimethylol-5,5-dimethylhydantoin (CAS RN: 6440-58-0)
- Bronopol (CAS RN: 52-51-7)
- Tris(hydroxymethyl)nitromethane (CAS RN: 126-11-4)
- Polyethylene glycol sorbitan monolaurate (CAS RN: 9005-64-5)
- Poly(acrylamide, trimethylaminoethyl methacrylate chloride) (CAS RN: 35429-19-7)
SALES SPECIFICATION
APPEARANCE
White to slightly yellow crystals or crystalline powder
99.0% min
MOISTURE
0.5% max
10ppm max
Hazard Symbols: XN N, Risk Phrases: 21/22/37/38/41/50/53, Safety Phrases: 9/16/26/33/37/39/60/61
GENERAL DESCRIPTION OF DISINFECTANT
- Mercuric Chlorides
- Formaldehyde
- 8-Hydroxyquinoline
- Copper Hydroxide
- Cresol
- Alcohols
- Iodines / Iodophors
- Chlorine releasing compounds
- Gluteraldehyde
- Phenolics
- Quaternary Ammonium Compounds
PRICE INFORMATION