PRODUCT IDENTIFICATION
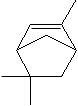
7785-70-8 (+)-alpha-Pinene
7785-26-4 (-)-alpha-Pinene
CLASSIFICATION
Flavor, Monoterpene
EXTRA NOTES
beta-Pinene is also available; used in manufacture of camphor, insecticides, solvents, plasticizers, perfume bases, synthetic pine oil; can be irritating to skin, mucous membranes; an insect attractants.
PHYSICAL AND CHEMICAL PROPERTIES
-62 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
33 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - alpha-Pinene
PubChem Compound Summary - alpha-Pinene
KEGG (Kyoto Encyclopedia of Genes and Genomes) - alpha-Pinene
http://www.ebi.ac.uk/chebi/ - alpha-Pinene
http://www.ncbi.nlm.nih.gov/ - alpha-Pinene
Local:
Turpentine is a semi-fluid substance consisting of two principal components, spirits of turpentine (volatile portion also known as oil of turpentine or turps ) and a type of rosin (nonvolatile portion also known as colophony). Turpentine is exuded from coniferous trees. The crude turpentine is distilled through steam into commercial turpentine, oil of turpentine. The pure turpentine oil is a transparent oily liquid with a penetrating odor and a characteristic taste. Turpentine is not a pure substance but a complex mixture of terpenes, particularly large proportion of pinene (bicyclic monoterpenic hydrocarbon), a compound from which camphor is manufactured. Terpene is a class of naturally occurring unsaturated hydrocarbons whose carbon skeletons are composed exclusively of isoprene C5 units (CH2=C(CH3)-CH=CH2), consisting of five carbon atoms attached to eight hydrogen atoms (C5H8). The stepwise distillation with water and carbonates yields terpenes. The most abundant monoterpene is pinene. There are two isomerers called alpha (or beta) pinene. The alpha form boils at 156-160 C, and of the beta form boils at 164-169C. It is used as solvents for paints, coatings and wax formulations. It is used as an intermediate for resins and for camphor, menthol, campholenic aldehyde and terpineol. It is used as lube-oil additives. Myrcene,one of the most important chemicals used in the perfumery industry, is obtained from beta-Pinene through pyrolysis.
APPEARANCE
COLOR, APHA
50 max
GENERAL DESCRIPTION OF TERPENE
|
Class |
number of isoprene |
Examples |
|
Hemiterpene |
1 (C5H8) |
Found in associated with Alkaloids, Coumarins and Flavonoids. |
|
Monoterpenes |
2 (C10H16) |
Geraniol, Citronellol, Pinene, Nerol, Citral, Camphor, Menthol, Limonene, Thujone |
|
Sesquiterpenes |
3 (C15H24) |
Nerolidol, Farnesol |
|
Diterpenes |
4 (C20H32) |
Phytol, Vitamin A1 |
|
Triterpenes |
6 (C30H48) |
Squalene |
|
Tetraterpenes |
8 (C40H84) |
Carotene (Provitamin A1) |
|
Polyterpenes |
> 10 (C5H8)n |
|